|
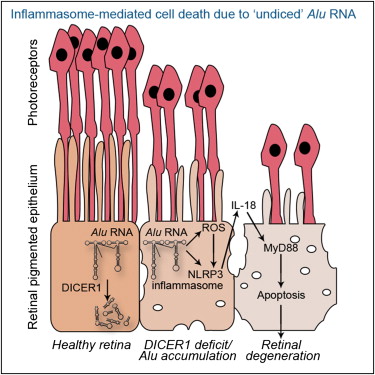
Alu RNA accumulation due to DICER1 deficiency activates NLRP3 inflammasome in RPE. Pharmacological inhibition of the inflammasome, MyD88, or IL18 prevents degeneration. Alu RNA induced RPE degeneration via mitochondrial ROS production, IL18, and MyD88. RPE in human geographic atrophy eyes display evidence of NLRP3 and MyD88 activation
|
|
Vascular Heterogeneity in the EyeThe main goal of the laboratory is to elucidate molecular mechanisms that fundamentally regulate vascular growth in normal and diseased states in the eye. In 2006, we answered the foremost outstanding question in vascular biology: "What is responsible for avascularity of the cornea?" This work (Ambati et al., Nature 2006) demonstrated that, contrary to prevailing dogma that a multitude of anti-angiogenic molecules was required for corneal avascularity, a single protein - soluble VEGF receptor-1 - was uniquely responsible. This work was hailed by Science as a “Signaling Breakthrough of the Year” (Adler et al., Science STKE 2007), and is far-reaching because the cornea is the default platform for testing therapies for cancer, atherosclerosis, and other diseases driven by angiogenesis. In 2009 this work was extended to the lymphatic vasculature with our discovery and characterization of a similar, soluble, splice variant of VEGF receptor-2 (Albuquerque et al., Nature Med 2009) which sequesters VEGF-C and is responsible, specifically, for the alymphatic cornea.
|
|
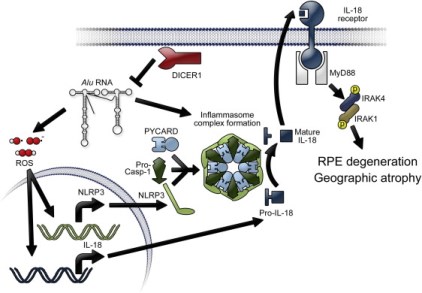
Alu RNA induces priming of NLRP3 and IL18 mRNAs via generation of ROS. Activation of the NLRP3 inflammasome triggers cleavage of pro-IL18 by activated Caspase-1 to mature IL18. IL18 signals via MyD88 to phosphorylate IRAK1 and IRAK4, which leads to RPE cell death.
|
|
The Interface of Immunity and Vascular Response in the EyeHow does the omnipresent immune system induce pathologic vascular responses in the eye? Given the fundamental lack of knowledge on the molecular basis of immune regulated angiogenesis, our lab is committed to unraveling these complex biologic interactions. We recently identified (Kleinman et al., Nature 2008) the immune receptor TLR3 as a surprising signaling receptor for small interfering RNAs (siRNAs) in a sequence-independent manner. We showed that siRNAs "generically" suppress angiogenesis in multiple mouse models by activating cell surface TLR3 rather than by triggering RNA interference. These findings, which were covered in commentaries by Nature Medicine (2008) and Nature Biotechnology (2008), advance the nascent understanding of the multifunctional aspects of the immune system within the eye. We will continue our investigations into immunovascular biology with the goal of mapping this critical interface while creating more effective and tolerable medicines to treat neovascular diseases.
|
|
Our lab made a major breakthrough in this field by identifying CCR3, a chemokine receptor which has been implicated to be a key player in allergic inflammatory processes, as a biomarker and potential therapeutic target for choroidal neovascularization in age related macular degeneration (Takeda et al., Nature 2009). Of special importance, we were able to detect abnormal blood vessels in vivo in the eyes of mice that spontaneously develop CNV, prior to invasion of the retina, by attaching anti-CCR3 antibodies to tiny semiconductor nanocrystals, or "quantum dots." The discovery of this target may some day enable detection and treatment of neovascular age-related macular degeneration before blood vessels invade the retina and damage eyesight.
|